Research
In November 2012, we set up a new laboratory at Kyoto Prefectural University with a mission to elucidate the molecular basis of nutrition and metabolism. In our Molecular Nutrition laboratory, we are focusing on ‘Foods and Metabolism’. Our studies have focused on the pathophysiological implications with regard to the regulation of nutritional adaptation, energy metabolism, and cancer. Our aim is to better understand the molecular mechanisms underlying metabolic syndrome and its associated diseases, and to establish novel strategies for the prevention of metabolic syndrome, and for fundamental basis of functional foods.
Skeletal muscle, as a target organ for metabolic syndrome study
A well-balanced body energy budget controlled by limitation of calorie uptake and/or increment of energy expenditure that is typically achieved by proper physical exercise is most effective against obesity and diabetes mellitus. Skeletal muscle is the largest organ in the human body and plays important roles in exercise, energy expenditure and glucose metabolism. Thus, we are focusing on skeletal muscle as a target organ, of our metabolic syndrome study. The mass and composition of skeletal muscle is critical for its function, and is regulated in response to changes in physical activity, environment or pathological conditions. For example, being confined to bed for a couple of weeks causes a marked reduction in skeletal muscle mass, called muscle atrophy. Currently, we are examining the detailed mechanism of muscle atrophy and energy metabolism, in vivo and in vitro, with gene modifications, especially focusing on nuclear hormone receptors and related cofactors.
Member
Professor
Yasutomi Kamei Ph.D
Associate professor
Tohru Saeki Ph.D
Graduate students
| (D3) | Yukino Hatazawa |
|---|---|
| (M2) | Yuma Hirose |
| (M1) | Takumi Onishi |
| (M1) | Takuya Yoshioka |
Undergaduate students
| (B4) | Kaho Takigawa |
|---|---|
| (B4) | Kei Maekawa |
| (B4) | Rintaro Matsuda |
| (B4) | Runa Yoshida |
Curriculum vitae
Name: Yasutomi Kamei
Education: Ph. D. (Agriculture), Kyoto University, Japan (March 1994)
Thesis title:
Studies on the mechanism regulating the formation of adipocytes by vitamins A and D.
Affiliation:
July 1994~ Postodoctoral fellow, University California San Diego. (CA, USA)
Identification of cAMP responsive element-binding protein (CBP) as a co-factor of nuclear hormone receptors.
October 1997~ Researcher, Osaka Bioscience Institute (Osaka, Japan)
Cloning of novel nuclear hormone receptor co-factor PGC1beta and its analysis as a stimulator of mitochondrial fatty acid beta-oxidation.
September 2001~ Researcher,National Institute of Health and Nutrition (Tokyo, Japan)
Finding of FOXO1, a forkhead type-transcription factor, as a regulator of skeletal muscle atrophy.
April 2005~
Associate Professor, Tokyo Medical and Dental University (Tokyo, Japan)
Study of inter-organ networks in metabolic organs, such as adipose tissues, skeletal muscles and liver, as target for metabolic syndrome
November 2012~Present
Professor, Laboratory of Molecular Nutrition, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University (Kyoto, Japan)
Rewards:
Young Scientist Award, by Japanese Minister of Education, Culture, Sports, Science and Technology (April, 2006)
Young Investigator Award, by Japanese Society of Nutrition and Food Science. (May 2006)
Young Investigator Award, by Japanese Society of Obesity. (October 2012)
Author of the most cited papers: From 1995 to 2000, his papers were highly cited (1,293 times) and ranked among the top seven most cited of all life-science researchers in Japan (January 2002, Newspaper of Japanese Economy and Industry, Nikkei Business Daily)
Mailing address
Yasutomi Kamei
Laboratory of Molecular Nutrition
Graduate School of Life and Environmental Sciences
Kyoto Prefectural University
1-5 Hangi-cho, Shimogamo, Sakyo-ku Kyoto 606-8522 Japan
Tel/Fax: +81-75-703-5661
E-mail: kamei@kpu.ac.jp
Summary of previous and ongoing projects:
Biological roles of nuclear receptor cofactors.
Fat-soluble hormones, including food-derived vitamins A and D, regulate complex programs of gene expression via nuclear receptor family of transcription factors. Recent studies, including those of Dr. Yasutomi Kamei, have led to the identification of cofactor protein molecules that appear to play important roles in mediating transcription by members of the nuclear receptor family. Dr. Kamei showed that CREB binding protein (CBP) could function as a nuclear receptor cofactor, and integrate various cellular signaling. Moreover, Dr. Kamei investigated the biological roles of cofactor proteins, focusing on skeletal muscle, adipose tissue and liver, which are important organs for maintaining the energy balance in the human body. Dr. Kamei showed that a nuclear receptor cofactor, PGC-1b can bind and activate orphan estrogen receptor-related receptors (ERRs). Transgenic mice overexpressing PGC-1b exhibit elevated energy expenditure, and are resistant to obesity induced by a high-fat diet or by a genetic abnormality. Furthermore, Dr. Kamei showed that another cofactor FOXO1 negatively regulates skeletal muscle mass and type I fiber gene expression, and leads to impaired skeletal muscle function. Activation of FOXO1 appears to be involved in the pathogenesis of muscle atrophy. These results provide insight into the molecular basis and will help develop new methods for treating lifestyle-related diseases, such as obesity and diabetes mellitus.
Current and future projects:
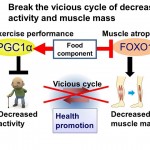
Muscle atrophy, due to bed rest or aging, decreases quality of life. To establish a method for preventing this, understanding the underlying molecular mechanisms is essential. Exercise enhances muscle function, acts on the whole body, and is effective for the prevention/improvement of metabolic diseases and depression. FOXO1 and PGC1α are transcriptional regulators that cause atrophy and increase exercise performance, respectively. In this study, the target genes of FOXO1 and PGC1α will be identified, and their molecular mechanisms and functions analyzed. Muscle atrophy and improved exercise performance as well as the effects of food components will be explained in terms of gene regulation. Thus, we will focus on FOXO1 and PGC1α, search for their target genes, analyze in vivo/in vitro phenotypes of animals/cells with modifications of these genes, and analyze their associated molecular mechanisms for preventing muscle atrophy and improving exercise performance (Fig. 1).
This study aims to clarify the molecular mechanisms underlying the atrophy associated with bedrest and aging, as well as exercise performance. This will be important for the fields of nutrition, exercise physiology, and metabolic disease. Aging-based decreased muscle mass and dysfunction (sarcopenia) reduce quality of life in elderly people and are serious health problems for aging societies. In the US, atrophy is observed in more than 20% of 60-year-old and 50% of 80-year-old people, and the medical cost of sarcopenia is huge ($18.5 billion in 2000). The muscle cell culture system used in this study is applicable for screening food components that could prevent atrophy and enhancing muscle function, potentially leading to the development of novel functional food and drugs.
Fig. 1. Schematic diagram of research Aging decreases muscle activity, leading to decreased muscle mass. Muscle atrophy causes muscle dysfunction and even more decreased activity. In this study, we will focus on FOXO1 and PGC1α and obtain fundamental information important for modulating their activity, stopping this vicious cycle, preventing muscle dysfunction, and improving quality of life.
A well-balanced body energy budget controlled by limiting calorie intake and/or increasing energy expenditure, which is typically achieved by appropriate physical exercise, is most effective against obesity and diabetes mellitus. Skeletal muscle is the largest organ in the human body comprising approximately 40% of the total body weight, and plays important roles in exercise, energy expenditure, and glucose/amino acid metabolism. Thus, we are focusing on skeletal muscles as a target for our study.
Skeletal muscles deposit energy in the form of protein (amino acid). They plastically adapt to their environment, and appropriate exercise with sufficient nutrition increases muscle mass. Meanwhile, various life-conditions, such as bedrest, aging, cancer, and other diseases causes muscle atrophy, which decreases energy expenditure (leading to obesity), decreases glucose uptake/increases blood glucose (diabetes), and decreases quality of life. In aging societies, a growing situation in many developed countries, including the US and Japan, the prevention/cure of atrophy is particularly important. The beneficial effects of exercise occur not only in skeletal muscles but also in other organs. Understanding the molecular mechanisms underlying muscle metabolism during exercise and hypertrophy/atrophy is important for developing methods to prevent muscle atrophy/dysfunction, which seriously impairs human health and quality of life.
FOXO1 is a forkhead-type transcription factor that antagonizes insulin-mediated anabolic signals. We have been investigating the role of FOXO1 in metabolic regulation in skeletal muscles. Specifically, based on the data that FOXO1 gene expression is induced by energy deprivation (Kamei et al., 2003b), we made transgenic mice overexpressing FOXO1 (FOXO1 Tg mice), which showed muscle atrophy (Kamei et al., 2004, Fig. 2). In addition, we showed that FOXO1 suppressed fat synthesis and glucose uptake (Kamei et al., 2008). Moreover, we showed that FOXO1 activates the genes involved in protein degradation (Yamazaki et al., 2010) and glutamine synthesis (possibly for ammonia clearance during protein degradation) (Kamei et al., 2014). These findings suggest that FOXO1 in skeletal muscles plays an important role in muscle atrophy as well as in the adaptation to energy deprivation. Clarifying the molecular mechanisms of FOXO1 in skeletal muscles may provide clues for developing methods to prevent muscle atrophy.
Besides FOXO1, the transcriptional coactivator PGC1α is important for muscle metabolism. PGC1α expression is increased in skeletal muscles by exercise (Miura et al., 2008). We have shown that PGC1a caused an increase in mitochondria number in skeletal muscles (Fig. 3) and increased energy expenditure (b-oxidation) and red/slow fiber formation (Kamei et al., 2003a). In addition, increased PGC1α leads to prolonged exercise performance (duration for which running can be continued) and at the same time, increases the expression of BCAA metabolism-related enzymes and genes that are involved in supplying substrates for the TCA cycle (Hatazawa et al., 2014, Hatazawa et al., 2015)
We recently created mice in which PGC1α is knocked-out specifically in skeletal muscles (PGC1α KO mice), which show decreased mitochondrial content. As PGC1α expression is decreased during periods of inactivity (such as bedrest and aging), our PGC1α KO mouse serves as a potential model of muscle inactivity. Generally, exercise acts on the whole body as well as skeletal muscles. PGC1α may mediate its beneficial effects such as improving metabolic diseases.
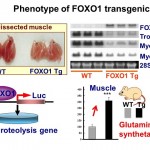
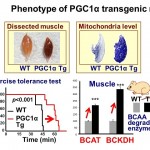
Fig. 2. (Left) Phenotype of FOXO1 transgenic mice: FOXO1 causes muscle atrophy Skeletal muscles of FOXO1 Tg mice are smaller and paler. Reduction in gene expression of type I fiber (red, slow type) proteins was observed in FOXO1 Tg mice. In FOXO1 Tg mice, glutamine synthetase mRNA was increased. FOXO1 activates genes involved in proteolysis. Thus, FOXO1 causes atrophy. WT, wild-type.
Fig. 3. (Right) Phenotype of PGC1α transgenic mice: PGC1α increases exercise performance Skeletal muscles of PGC1α Tg mice are red in color, with increased expression of mitochondrial enzyme succinate dehydrogenase (dark blue in histochemical analysis). Exercise tolerance test: PGC1α Tg mice showed increased capacity for longer treadmill running. In PGC1α Tg mice, BCAA degradation enzyme (BCAT, BCKDH) mRNA was increased. BCAT, branched chain amino transferase; BCKDH, branched chain α-keto acid dehydrogenase; WT, wild-type. This study is collaboration with Dr. Miura (Univ of Shizuoka) and Dr. Tadaishi (Tokyo Univ of Agri.).
Currently, we are examining the detailed mechanisms of muscle metabolism in vivo and in vitro, focusing on gene regulation, particularly transcriptional regulators. To date, few trials have investigated, via gene regulation, muscle atrophy and increased exercise performance, as well as the effects of food components on them. This will be a novel study to analyze the molecular mechanisms involved, focusing on FOXO1 and PGC1α as major regulators.
Access
Address
1-5 Hangi-cho, Shimogamo, Sakyo-ku Kyoto 606-8522 Japan
Phone Numbers
Kyoto Prefectural University:+81-75-703-5101